
200X concentrate of whole Oregon Cranberry fruit
15% A-Type PAC’s tested by BL-DMAC method
1.The Science Behind Cranberex®
1.a - Background
North Americans have used cranberries for centuries as a valuable and nutritious food source, but in recent years cranberry’s therapeutic use has focused primarily on the ability to support urinary tract health, particularly for those prone to recurrent UTI’s.
1.b – Cranberry’s active constituents
The constituent in cranberry responsible for its therapeutic effect was for long a matter of speculation. What made cranberry different from the range of similar tannin-rich berry fruits? Today scientific consensus is that cranberry’s activity is due to its unique proanthocyanidins (PAC’s) containing the ‘A-type’ linkages, not found in PAC’s from other sources [1]. Cranberex® is standardized to a high level (15%) of these ‘A-type’ linkage PAC’s.
1.c – Potency assay methods
The accurate measurement of total Cranberry A-Type PAC’s has presented a challenge, due to their structural complexity and degree of polymerization. A number of analytical methods, such as: colorimetric, chromatographic and MS have been used. However the results produced by these methods are variable, difficult to reproduce, and don’t distinguish clearly between PAC’s from Cranberry and other sources.
1.d – The BL-DMAC method
In 2010 however, the BL-DMAC method for quantifying the A-Type PAC’s in Cranberry was published. This method has now been endorsed by a number of international labs and authorities, including the USDA and Rutgers University. It was also used for validating cranberry UTI claims in the EU [2]. This DMAC method now allows health claims to be made that correspond most accurately to specific amounts of Cranberry PAC’s. This is the method used for testing A-Type PAC’s in Cranberex®.
1.e – Anti-Adhesion Activity (AAA) of cranberry
Cranberries support UTH in a unique way: the A-Type PACs inhibit the adhesion of E.coli bacteria to walls of the urinary tract. Studies have confirmed that this effect is specific to the A-linked PAC’s (cranberry), but not to other PAC rich foods with the more common B-type linkages (e.g. chocolate and grapes) [1].
As this bacterial anti-adhesion mechanism does not kill bacteria, there is less chance of selection for resistant bacterial strains. This has been considered as an advantage compared to antibiotics treatment for UTI’s and in some cases more effective in reducing the re-occurrence of UTI’s [3].
2. The Clinical Studies that support Cranberex®
2.a – Verified Potency
Cranberex® contains 15% PAC’s, as tested by the BL-DMAC method. This makes it one of the most concentrated cranberry extracts available in the North America or Europe. This potency and standardization connects directly with the dosages established in the key clinical trial that established the link between A-Type PAC’s and anti-adhesion activity [4].
Tests that compared PAC levels in Cranberex® with five leading finished product brands, showed a far greater potency in Cranberex™ (see fig 1).
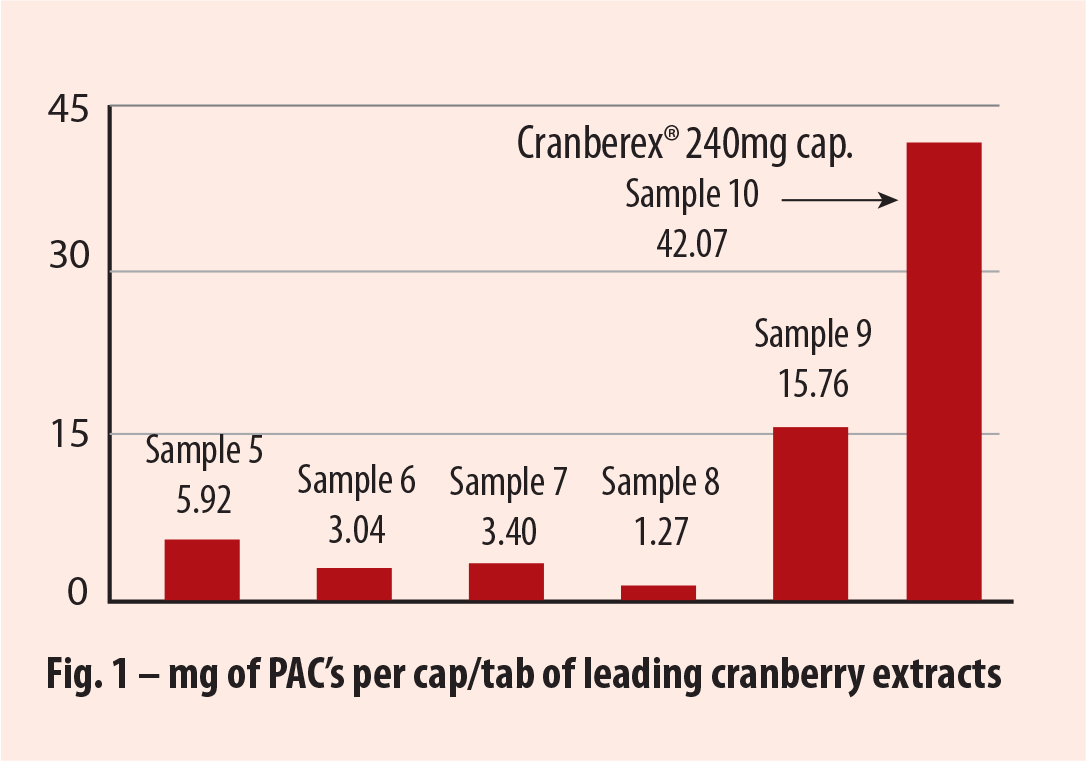 |
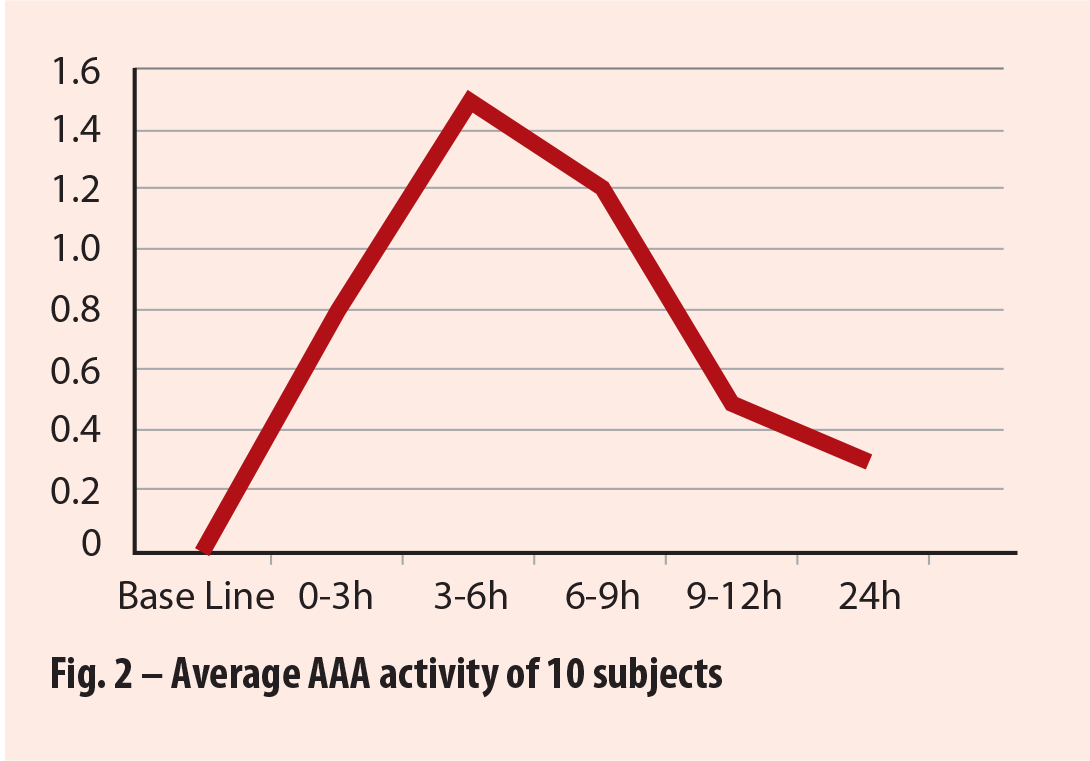 Source: Rutgers University, Feb 2017, Bacterial Anti-adhesion Activity of Human Urine: Cranberex (Ethical Naturals) |
2.b – In-Vitro Anti-Adhesion Activity (AAA) data from Rutgers University*
This in-vitro study compared the AAA of Cranberex® with nine other brands of cranberry extract (raw material and finished products). In this study, Cranberex™ met the anti-adhesion criteria at a concentration of only 0.23mg/mL. The next most potent brand required a concentration of 60mg/mL to achieve the same anti-adhesion result. The study concluded that Cranberex™ had excellent anti-adhesion activity.
2.c – Ex-Vivo AAA study from Rutgers University*
This study, was designed to test actual anti-adhesion activity in the urine after consumption of two doses (1 dose in the evening and 1 dose in the morning) of Cranberex® (at recommended dosage level) in human participants. The report states that AAA response increased rapidly in all participants 3 – 6 hours after consumption of Cranberex™. Half of the participants achieved 100% or significant bacterial Anti-Adhesion Activity, while half achieved 50%, resulting in an average of 75% AAA. (See fig 3).
2.d – Cranberex™ Dosage Level
The recent, multicentric randomized, double blind clinical trial clearly affirmed, for the first time, a bacterial anti-adhesion effect in urine, based upon 36mg/day of cranberry PAC (by BL-DMAC method) [4]. It also reported that effectiveness was dose-dependent, prolonged up to 24 hours with 72 mg of PAC, and increasing with the amount of PAC (by BL-DMAC method) consumed.
Cranberex™ is standardized to 15% PACs, and provides these dosages with consumption of 480mg (72mg PAC) and 240mg (36mg PAC) respectively.
* Copies of these tests and studies are available upon request
3. Why The Power of Oregon Cranberry?
The U.S. produces over 90% of the world’s Cranberry crop, and about 85% of this is produced in the North East (primarily Wisconsin and Massachusetts).
However a small percentage of U.S. Cranberry is grown in the cool coastal areas of Oregon, and this climate produces some special qualities in the harvested fruit: the key anthocyanin content in Oregon grown Cranberry is significantly higher than in fruit harvest in the North East: Oregon (70mg/100g), Massachusetts (42mg/100g) and Wisconsin (37mg/100g) [5]. This high content of therapeutic constituents is why the source of Cranberex™ begins on the Oregon coastline.
4. The Need for Premium Clinically Tested Cranberry Extracts
UTI’s are one of the most common bacterial infections accounting for 10.5 million visits to doctor’s offices each year, and costing an estimated $3.5 billion per year in the US alone [6]. Some estimates place the percentage of antibiotic resistant cases at up to 10% of this number. As a result, many people are looking for alternative, naturally based ways to support UT Health. The science behind Cranberex® makes it ideal for those seeking health based solutions.
Encapsulated and Bottled Finished Products:
Save your time and money through our in-house manufacturing services, certified to FDA 21 CFR-111 and FSMA compliance by NSF
Through our vertically integrated supply chain, testing lab and manufacturing facility ENI can provide you with the ultimate in convenience and confidence for your branded Cranberex® stand alone, and special formulas. This vertical integration also allows us to offer an integrated cost advantage directly to your company, along with the confidence of fully certified manufacturing.
From Field to Finished product®
The above statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or mitigate and disease.
References:
[1] Howell, A.B., et al. (2005). A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 66, 2281–2291.
[2] Prior, R. L. et al. (2009). Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. (www.interscience.wiley.com) DOI 10.1002/jsfa.3966.
[3] Kaspar, K. L., et al (2015). A randomized, double-blind, placebo-controlled trial to assess the bacterial anti-adhesion effects of cranberry extract beverages. Food Funct 6 (4):1212-7.
[4] Howell, A. B. et al (2010). Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infectious Diseases. Retrieved on 7/11/2016 from ncbi.nlm.nih.gov
[5] American Herbal Pharmacopoeia and Therapeutic Compendium (2016). Cranberry Fruit, Vaccinium macrocarpon Aiton, Revision. Page 15.
[6] Flores-Mireles, A,L., et al. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiology 2015 May. 13(5) 269-284



